Chemistry

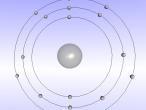
Lithium Atom
This blog will discuss the shape and characteristics of lithium atoms. Some questions and will be asked further in the investigation for you to answer yourself.
NaCl Lattice Blog Entry FINAL (Ryan Gray ASC091C)
By Ryan Gray ASC091C
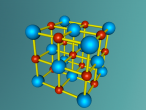
This model is of a salt crystal lattice, with the chemical formula NaCl. Its different properties allow it to have many uses in society, including as a preservative, flavouring and currency. Salt demonstrates ionic bonding and its structure makes it interesting to model, because the crystal lattice can continue on forever and doesn't have a specific number of atoms like water does, with each water molecule consisting of one oxygen and two hydrogen atoms. For the purposes of this project, I limited my model to a 3x3x3 cube so it could be easily seen. The distance between the atoms was also expanded to allow the bonds to be more clearly viewed.

HydROgEn cYAnIde
Atoms are the building blocks of all matter that we can touch, affect and be affected by. They each contain varying numbers of protons, electrons and neutrons that make up hundreds of thousands of different elements, molecules and compounds used both by nature and humans. These can range from a simple oxygen molecule (O2) to more complicated compounds such as Methamphetamine, C20H15N. One such molecule is HCN; Hydrogen Cyanide.
LITHIUM 9D
Boron Atom
Boron Atom (By Melody Suen)
Acetone Blog
My name is Michaela Cheong and I am a student at Brisbane State High School. In our current unit of science, we are learning about chemistry, specifically the structure and characteristics of atoms (building blocks which make up everything) and compounds (consist of two or more atoms which are chemically bonded). In this blog, I will show you a 3D model of a molecule of acetone along with a discussion of it’s molecular structure, uses and properties.