NaCl Lattice Blog Entry FINAL (Ryan Gray ASC091C)
By Ryan Gray ASC091C
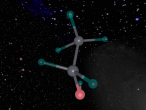
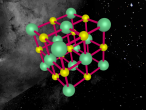
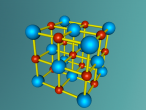
This model is of a salt crystal lattice, with the chemical formula NaCl. Its different properties allow it to have many uses in society, including as a preservative, flavouring and currency. Salt demonstrates ionic bonding and its structure makes it interesting to model, because the crystal lattice can continue on forever and doesn't have a specific number of atoms like water does, with each water molecule consisting of one oxygen and two hydrogen atoms. For the purposes of this project, I limited my model to a 3x3x3 cube so it could be easily seen. The distance between the atoms was also expanded to allow the bonds to be more clearly viewed.
The NaCl lattice consists of a cube, where each chlorine atom is surrounded by 6 sodium atoms - two in each dimension - and vice versa. Because of this, it is known as 6:6 coordinated. It bonds in this way because of the number of electrons in the outer shells of both the chlorine and sodium atoms. Chlorine has 1 electron missing in its outer shell, and sodium has 1 electron in its outer shell, so when they bond molecule formed becomes much more stable. Because the attraction between positive and negative ions (an atom that has a different number of electrons to protons, with the positive/negative referring to its charge) releases more energy, and that the amount of energy released is correlated with the molecule's stability, the NaCl lattice has the maximum number of chlorine atoms touching the sodium atom without any chlorine atoms touching each other. This makes it a very stable molecule, and also accounts for some of its properties and uses.
The electrostatic forces between the Na and Cl atoms are quite high, meaning that a lot of energy is needed to overcome the bonds. This results in very high melting and boiling points for salt, meaning it can be used safely in cooking. It is also quite brittle - a small shift in the structure and sodium atoms will be touching sodium atoms, making it much less stable and causing repelling within the crystal lattice. It has a hardness of 2.5 on the Mohs scale, between gypsum and calcite, and it's weight is 58. Sodium chloride is soluble in water as well.
Salt has had many uses throughout history, most notably as a flavouring but also as a currency and preservative. In ancient times, cakes of salt were used as money in Ethiopia and Tibet. In Rome, an allowance of salt was given to officers and troops, which could be converted into money. One of the most ancient roads in Italy was known as the 'Salt Route' over which salt was traded between provinces in the country. Later in history, salt has been and continues to be used as a preservative for meat, fish and other foods. When salt is added to snow and ice, it reduces the melting point of the substance, so it is also used as a way to clear snow off roads and driveways.
The links below gave me information about salt's structure and properties. The first one was particularly useful in explaining how salt's chemical makeup caused it to have the properties it does.
https://www.britannica.com/
http://www.chemguide.co.uk/
One of the questions I am still interested in is how a lattice ends. Most websites only mention how the lattice can extend for billions or trillions of atoms, and they don't describe how the structure can stop extending. Because of the nature of the atoms in salt, there has to be an equal number of sodium and chlorine atoms to maintain stability. But that could mean that a crystal can't end on an odd number of rows - the number of atoms of each type would differ by 1, unless there was an extra atom outside the cube, only connected by 1 bond. If anyone knows how the expanding stops, please reply in the comments, because it could mean my model is inaccurate!
One of the most difficult aspects of programming the model was keeping the code concise. At first, it seems like the code could be compacted into a procedure for a layer, and that procedure could be repeated three times. But because the sodium and chlorine alternate, the second layer is different to the top and bottom layers, so two slightly differing procedures had to be made. After these three layers were implemented, a final set of bonds could be added to connect the different layers. Aside from this, the programming was relatively simple and could be achieved in a step-by-step manner, running the program then finding the next step to continue. Sometimes identifying whether the turtle should roll, tilt or turn was difficult, but it didn't take too long to find my errors. Also, when moving up a layer, the settings on the rotation, tilt and turn were skewed, but they could be reset using the code reset home.
By Ryan Gray ASC091C
Here is the 3D model (again)
Below is the Logo code: