Ethanol Molecule

Ethanol is a molecule containing a mere 9 atoms of which there are 3 different atoms and has been known to human long prior to the advent of modern chemistry. These three atoms are hydrogen (6), carbon (2) and oxygen (1). Ethanol, more commonly known alcohol is the primary alcohol found within alcoholic beverages such as beers, wines and spirits. It is a neurotoxic, psychoactive drug and has been used for recreation since the early ages. In addition it has various everyday uses ranging from cleaning products, fuel for table top cookeries, solvents, industrial applications as well as it being utilised in medicine. Ethanol is also known o be found within thermometers where it is coloured red.
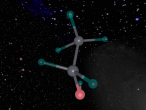
Ethanol's chemical formula is C2H6O. This chemical formula can also be written as CH3CH2OH or C2H5OH. It is made of nine atoms that include two carbon (C) atoms, six hydrogen (H) atoms, and one oxygen (O) atom. Carbon is the 15th most abundant element in the Earth's crust and the fourth most abundant element in the universe by mass after hydrogen, helium, and oxygen. Oxygen is a chemical element with symbol O and atomic number 8. It is a member of the chalcogen group on the periodic table and is a highly reactive non-metal and oxidizing agent that readily forms oxides with most elements as well as other compounds. Hydrogen is a chemical element with chemical symbol H and atomic number 1. With an atomic weight of 1.00794 u, hydrogen is the lightest element on the periodic table.
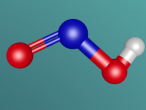
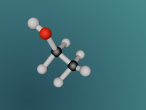
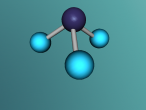
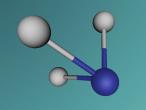
Its chemical structure is illustrated in the following picture. Here, there is a methyl group (which is the CH3-), a methylene group (which is the -CH2-), and a hydroxyl group (which is the -OH) in the chemical structure.

Ethanol is a clear, colourless liquid with a characteristic pleasant odour and burning taste. It is highly flammable. Ethanol is used to dissolve other chemical substances and mixes readily with water and many organic liquids. Ethanol is considered a volatile organic compound.Ethanol rapidly absorbs water from the air. It mixes readily with most organic liquids.
Melting Point: -114°C
Boiling Point: 78.5°C
Specific gravity: 0.8
Flash point: 9-11°C
I still wish too know the specifics of why and how the particular atoms in Ethanol bond and create molecules. Moreover, I wish to learn more about covelant bonds and there role in the boodning process. In addition, I wish to explore, Ethanol as a substance more and understand it workings and improtance in day to day life.
The following websites helped me in my research:
- http://www.bbc.co.uk/schools/gcsebitesize/science/aqa_pre_2011/oils/poly...
- http://study.com/academy/lesson/what-is-ethanol-formula-structure-uses.html
- http://www.rsc.org/chemistryworld/podcast/CIIEcompounds/transcripts/etha...
- http://www.ucc.ie/academic/chem/dolchem/html/comp/ethanol.html
- http://www.gcsescience.com/o36.htm
The programming was quite challenging at first and it is still quite so although to an extent. Programming is not my strong suit however themathematiccs and logistics wa quite graspable and once Ihad understood the the underlying concept, it was quite easy to create the Ethanol molecule. My partner was also very experienced in the digital programming field, so he was able to help me with my struggles. This experince has allowed me to understand the mathematics involved and has given me a unique perspecive of the relationship between science and digital technology.
The link to the 3D model is below:











Comments
Feedback
Ethanol is made up of 3 types of elements, not atoms.
You could have mentioned the atomic mass of ethanol and the type of bond between ethanol. is it covalent or ionic?
You have some spelling errors.
Feedback
Ethanol is made up of 3 types of elements, not atoms.
You could have mentioned the atomic mass of ethanol and the type of bond between ethanol. is it covalent or ionic?
You have some spelling errors.