Molecule

FINAL Methane Blog by Anna Lei (ASC092A)
The compound methane has the chemical formula of CH4. Methane is the simplest hydrocarbon of the paraffin series and a potent greenhouse gas, as well as being a large component of natural gas. The major human-associated source of methane is the production/combustion of coal (Britannica, 2016). In nature, methane is the product of anaerobic bacterial decomposition and certain human/animal activities (7% methane in flatulence). It can be found in wetlands, termites, landfills, volcanoes and oceans. The abundance of methane makes it a widely used fuel for heat and light (energy) production (ARM, 2016). In this blog, the details of methane will be uncovered.
- 2 comments
- Read more
- 4236 reads
ETHANE MOLECULE
Ethane
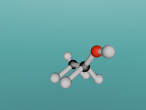
Ethane, otherwise known as C2H6, is an organic compound which is a colourless, liquefied gas. It provides many uses including those from the industrial and a consumer point of view. Ethane is used in fuel, fuel additives, ion exchange systems, paint additives, pigments, plasticizers, building/construction materials, fabric products, textile products, leather products, food packaging and much more.
H2O Molecule
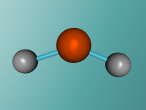
Imagine you are sitting with your feet in a pool (filled with liquid water), watching the steam (gaseous water) rise from the spa nearby as you sip a drink from your glass filled with ice cubes (solidified water). Not many other chemical substances can exist in all three states of matter within the same temperature range. In fact, water it is the only substance which naturally occurs in all types. This is why H2O (commonly called water) is one of the most interesting chemical compounds in the universe, as well as being essential for life! (We have never found any organism that doesn't contain H2O) Apart from being the most abundant compound on the Earth's surface, it has many other unique properties such as it's polar covalent bonds.
NaCl Lattice Blog Entry FINAL (Ryan Gray ASC091C)
By Ryan Gray ASC091C
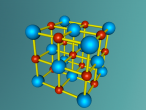
This model is of a salt crystal lattice, with the chemical formula NaCl. Its different properties allow it to have many uses in society, including as a preservative, flavouring and currency. Salt demonstrates ionic bonding and its structure makes it interesting to model, because the crystal lattice can continue on forever and doesn't have a specific number of atoms like water does, with each water molecule consisting of one oxygen and two hydrogen atoms. For the purposes of this project, I limited my model to a 3x3x3 cube so it could be easily seen. The distance between the atoms was also expanded to allow the bonds to be more clearly viewed.