Model
Phosphorus Atom Joey Stephens Brennan
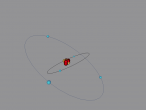
Phosphorus Atom
1. An element is a substance that cannot be broken down chemically or physically into a simpler substances. Elements are what makes up the world we live in, and each element has an atom with a different, definitive structure. Phosphorus is the fifteenth element found on the periodic table of elements. Using VRMath2, an online coding software, a 3D model of a phosphorus atom was constructed. The following blog will outline the structure, characteristics and composition of the atom, and explain any difficulties or interesting ideas found during the process of constructing the atom, as well as show an image of and provide a link to the model of the atom on the VRMath2 website.
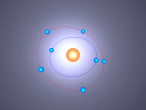
Boron Atom
Boron is the fifth atom in the periodic table, being a metalloid. The rare element, only made as compounds in the nature, has been used commonly for glass and glazes production. While its presence has been known since 1808, the first 99 percent pure boron was made only in 1909. A 3D model of this boron was made using the VRMath2.0, shown below. The Bohr model has been used to represent the element, as it simplifies the characteristics of electron movements. However, the model is not entirely accurate. In the process of programming the atom, many difficulties and challenges were experienced.
Nitrogen Atom
It is an element that is an essential to all life on Earth, yet in its purest form it can suffocate living organisms. Nitrogen is one of the most important elements on Earth – it can be found in all living systems and makes up around 78% of the Earth’s atmosphere. It is a constituent of protein and nucleic acids. In its gaseous form, nitrogen is colourless, odourless and generally considered inert. In its liquid form nitrogen is also colourless and odourless, and resembles water. Nitrogen was first recognised in 1772 by number of scientists; Carl Wilhelm Scheele, Daniel Rutherford, Henry Cavendish and Joseph Priestley, who all found that air was composed of three different gases, oxygen, carbon dioxide and another gas which they dubbed ‘foul air’ (nitrogen). Nitrogen was first recognised as an element by Antoine Laurent Lavoiser in 1786, and named nitrogen in 1790 by Antoine Laurent Lavoiser. Today, nitrogen is used in food preservation, explosives, and aids medical research.