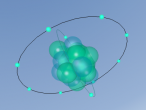
a blog about CHLORINE by Chanupa Amarasinghe
Have you ever driven a car?
Have u ever drank water straight from the tap?
Have you ever painted a wall?
If so, the chances are... you've been exposed to chlorine.
The element chlorine (Cl) is one of the 92 natural elements found on Earth (Euro Chlor, 2015). Chlorine is found in the 17th column of the modern periodic table as number 17. It was first discovered by Carl Wilhelm Scheele in 1774. According to Winter (n.d), he unknowingly obtained the element through a reaction between pyrolusite (manganese dioxide, MnO2) with hydrochloric acid (HCl, also known as muriatic acid). However, Scheele mistakingly believed the resulting gas contained oxygen, and was not a new element. 36 years later, in 1810, it was Sir Humphry Davy who confirmed chlorine to be an element and also named the element. Currently, chlorine is most commonly used as a defence against waterborne microbiological infections and thus is used to make drinking water safe for consumption and to treat swimming pools. In-addition, chlorine has many other uses as well, for example, in Europe, about a third of all produced chlorine is used for the synthesis of plastic PVC in objects such as doors, flooring and even car lamps (Euro Chlor, 2015). Apart from the above, chlorine can be used in medicinal practises, technological products and many more.
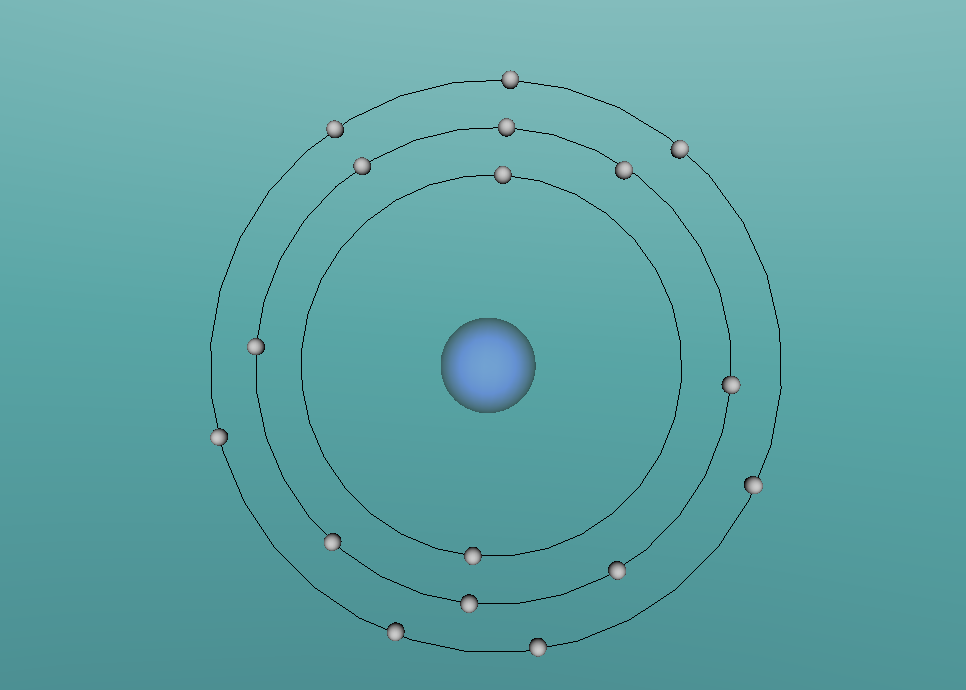
The pure chemical element, chlorine, has the physical form of a diatomic green gas (room temperature form), which is rarely found free in nature as it can combine with almost every other element. Because of this, chlorine occurs in nature mainly as common salt (NaCl), carnallite [ KMgCl2.6(H2O) ], and sylvite (KCl) (Lookchem, 2008). The name chlorine is derived from chloros, meaning green, referring to its gas form's colour (Lenntech, 2016). This gas is two and one half times heavier than air, is exceedingly poisonous, and has a strong suffocating odour. It acts as an irritant for the respiratory system and other mucous membranes. Chlorine's solid and liquid forms are powerful oxidising, bleaching, and disinfecting agents.
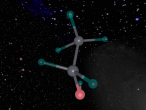
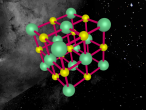
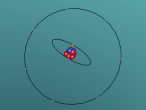
The chlorine atom, like all atoms, is comprised of protons and neutrons which make up the nucleus, and shells of electrons. The chlorine atom has an atomic number of 17, meaning it has 17 protons, which are found in the atom's nucleus. Also found in the nucleus are 18 neutrons. Surrounding this, are three sets of shells, which comprise of 17 electrons. The first shell (closest to nucleus) comprises of 2 electrons, followed by the second shell with 8 electrons and the outermost shell with 7 electrons (as shown in the model above). Chlorine has an atomic mass of 35.453 and a density of 3.214 grams per cubic centimetre (Blaszczak-Boxe, 2014). Furthermore, the temperature of which chlorine melts (melting point) is -101.5 degrees celsius while the boiling point is -34.04.
The following links will help further enhance your knowledge about chlorine:
http://www.livescience.com/28988-chlorine.html
http://www.rsc.org/periodic-table/element/17/chlorine
http://www.lenntech.com/periodic/elements/cl.htm
http://www.eurochlor.org/the-chlorine-universe/chlorine-in-nature.aspx
http://www.chemicalelements.com/elements/cl.html
After researching about chlorine, one question has arisen and has caught a great deal of my attention: "Since a low concentration of chlorine is used to purify water, wouldn't it eventually inflect effects on the human body?" I believe, this question will need to be further investigated upon before settling on a definite answer. If any readers do infact know the answer to my question, it would be highly appreciated if you leave the answer in the comments (below).
Above, a model of a chlorine atom may be seen. This was made using a software called "VRMATH2.0", Although this software is not superiorly advanced, it is still very useful when attempting to make a 3D object. At first, using the software seemed quite difficult but after a few test demos, it became fairly simple. A difficulty that was present during the creation of the atom was; the making of the chlorine nucleus. The protons and neutrons (inside the nucleus) were meant to be infused with each other, through a process called, "clumping". To do this, one must draw a 2d model of the nucleus then add layers over. However, due to lack of time and experience, this was unable to be done. Therefore, a general sphere represents the nucleus as a whole. Despite the one difficulty that occurred, using VRMath2.0 was a great experience as it certainly enhanced my capability of online programming and blogging.
To create your own 3D models, visit
https://vrmath2.net
By Chanupa Amarasinghe
Groups: