Geometry
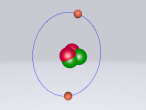
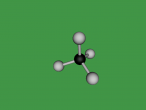
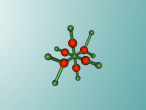
Methane Molecule
Atoms exist all around us, whether it be in living things, or in inanimate objects. They are the basic building blocks of life. However, an atom comprises of a proton, neutron and electron. Protons are positively charged sub-atomic particles, electrons are negatively charged and neutrons have no charge. Atoms can also combine with compatible atoms, to form molecules. Molecules can either just comprise of an element, one type of atom, or a compound, multiple types of atoms. A common molecule found in the air is methane. Methane is an green house gas that is the seventh most prevalent gas in the ozone layer. Methane is used for heat and light, utilising its flammability. Methane comprises of Carbon and Hydrogen. But how do we actually identify methane in the air? Well there are two ways; the composition and structure, and the characteristics.
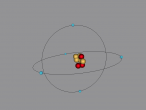
LITHIUM 9D
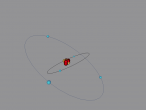
Boron Atom
Boron is the fifth atom in the periodic table, being a metalloid. The rare element, only made as compounds in the nature, has been used commonly for glass and glazes production. While its presence has been known since 1808, the first 99 percent pure boron was made only in 1909. A 3D model of this boron was made using the VRMath2.0, shown below. The Bohr model has been used to represent the element, as it simplifies the characteristics of electron movements. However, the model is not entirely accurate. In the process of programming the atom, many difficulties and challenges were experienced.
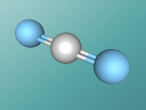
Aluminium Chloride
Compounds are substances which are formed when two or more chemical elements are chemically bonded together. Compounds are found everywhere, from the water (H2O) which we drink, to the aluminium chloride we use as a deodorant. They consist of various atoms arranged in a specific structure, called molecules. Compounds are formed through two types of chemical bonding, covalent and ionic bonding. A covalent bond is formed when electrons are shared between atoms, whereas ionic bonds are formed through the attraction of oppositely (positive charge and negative charge) charged ions.
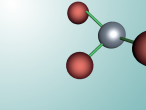
Calcium Chloride
Introduction
This blog will detail the composition, structure and characteristics of calcium chloride and includes
Boron Atom (By Melody Suen)
At the QUT workshop, I learnt how to use VRMath 2.0 to program, create 3D molecules/atoms and how to write a blog. With my partner Hana, we created a 3D model of a boron atom. In this blog post, I will explain the composition, structure and characteristics of boron, as well as the programming used.