Hydrochloric Acid
Everything contains atoms. Atoms are the basic units of matter and the defining structure of elements. Atoms are made up of three differently charged particles: positively charged protons, neutral neutrons and negatively charged electrons. Protons and neutrons are heavier than electrons and reside in the centre of the atom, called the nucleus.
Elements are the base conponents of everything. Elements are defined by the fact that they contain only one type of atom and make up what we call the Periodic Table. Oxygen is an atom as it contains only two Oxygen atoms and is thus called O2. Compounds on the other hand contain two or more different types of atoms which have been in some way chemically binded. an example of a common compound is table salt, NaCl as it contains 1 sodium atom, Na, and 1 chlorine atom, Cl, which have been chemically bonded together in a proccess known as ionic bonding.
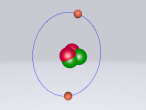
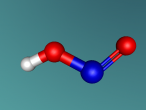
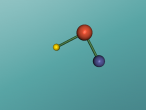
Hydrochloric Acid, Hydrogen Chloride, Muriatic Acid or HCl is a form of acid created by the chemical bonding of the two elements, Hydrogen and Chlorine. Acids are inorganic molecules that release hydrogen ions (positively charged hydrogen atoms) when added to water. when this happens the hydrochloric acid breaks apart or dissociate and the number of hydrogen ions released during the process determine the acidity of the solution. Acids come in two basic types weak acids and strong acids. If the acid dissociates partially it is a weak acid. If it dissociates fully, its a storng acid. Hydrogen chlorine is a strong acid and is bonded by a process called covalent bonding. This means that the hydrogen atom will share a pair of electrons with the chlorine atom in order to fill both their outer shells.
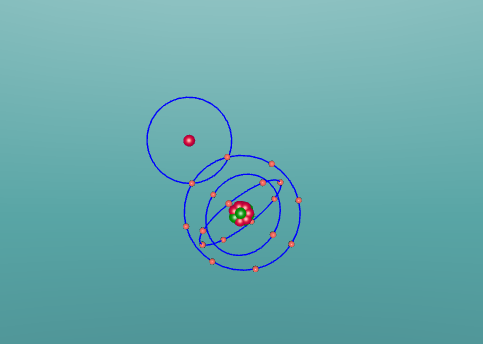
However once water is added the two atoms seperate which hydrogen ions becoming positively charged, creating the acidity, and chlorine ions becoming negatively charged.
Hydrochloric acid is a clear liquid but does have a strong odour and sour taste, common to most acids. It has a boiling point of 110 degrees celsius and is corrosive which means it corrodes biological tissue. As such swallowing or inhaling HCl causes many internal problems as well as physical contact. These include:
- Severe abdominal pain
- Severe chest pain
- Fever
- Drooling
- Pain in the mouth and throat
- Abrupt drop in the blood pressure
- Vomiting blood
- Swelling in the throat that causes difficult breathing
Hydrochloric acid is also found in the human body. It is naturally found in the gastric juices of your stomach. The role of hydrochloric acid, as well as the other gastric juices, is to break down foods and cause the release of enzymes to further aid this process. The gastric juices, together referred to as gastric acid, contains mostly potassium chloride (KCl) and sodium chloride (NaCl). As hydrochloric acid is such a corrosive chemical, it only makes up 5% of gastric juices. This gives gastric acid a very low ph range, usually around 1-2. HCl is secreted by the parietal cells in the stomach and since it is so strong, if there was no mucus lining on the stomach it would eat through it. Hydrochloric acid breaks down many of the foods we eat as well as fighting any bacterias which may enter the stomach in the foods we eat. it also plays a role in enzyme release and converts pepsinogen into pepsin which in turn dissolves proteins in the food we eat.
Creating this program I learnt many key skills which I can use later in life but I also faced many challenges such as creating the protons and neutrons to move as in a real atom these particles are constantly moving. After many hours I finally solved this problem by creating a proton and neutron world and then uploading them into my code as an 3xd file using a code I learnt from andy: [WORLD "/sites/default/files/user/u3/world/proton.x3d]. This allowed the protons and neutrons to vibrate.
Some questions I would like to find out is
What are the negative affects of hydrochloride acid in the body
Where are other places Hydrochloric acid is naturally found
And programming Questions
How do I make the hydrogen electron to show covalent bonding better
Can Hydrochloric acid bond with other elements to create stronger acidic compounds
Further Reading
http://www.livestrong.com/article/419261-role-of-hydrochloric-acid-in-the-stomach/
http://firstaidkelowna.ca/what-happens-if-hydrochloric-acid-is-swallowed/
http://study.com/academy/lesson/hydrogen-chloride-formula-structure-properties.html/
- Bwalt66's blog
- Login or register to post comments
- 6068 reads

















Comments
brayden please send the logo
brayden please send the logo code for this to me