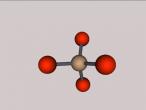
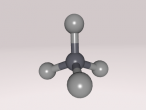
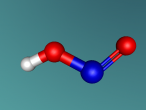
Nitrous Acid Molecule
A molecule is when two or more atoms join chemically; nitrous acid is one of many molecules. Nitrous acid, which its chemical formula is HNO2, is an unstable acid and only exists in solutions or nitrite salt. Interestingly, it is naturally produced by the Earth's atmosphere, and when it reacts with water (H2O) and nitric oxide (NO), it helps regulate ozone layer in the atmosphere, which is currently being damaged by human activities. If this acid comes in contact with human, it may affect respiratory health and cause irritation, as it is slightly toxic. In this blog, it will present: 3D model of the acid composition; structure and characteristics; further information and questions; intriguing ideas and difficulties found in programming; and links to the 3D model and logo program.
Its compositions are hydrogen, nitrogen and oxygen, all being a gas atom. Hydrogen (H), discovered by Henry cavendish, consists of one proton and one electron. It is the most common gas and non-toxic. As hydrogen is non-toxic, it may be a possible contributing factor that nitrous acid is not very as toxic as other acids. Nitrogen (N), part of amino acid, was discovered by Daniel Rutherford, who also found positively-charged nucleus. Making 80% of the Earth's atmosphere,it is unreactive, but when it comes in contact with hydrogen, it creates a reactive compound. Oxygen (O) is often defined as the "air" that we breathe in; however, considering the fact that it only makes up 21% of the Earth's atmosphere, it is not scientifically true. Compositions of nitrous acid makes HNO2.
Structure of this molecule is the arrangement of the compositions. Bonds between hydrogen, first oxygen atom and nitrogen are single; however, the bond between nitrogen and second oxygen atom is double. Due to a double bond in this structure, this acid can exist in two different isomers – cis or tran isomer. Additionally, first oxygen atom holds the hydrogen atom firmly, which forms a weak acid. Also, both oxygen atoms linking to nitrogen are electronegative. Structure of nitrous acid determines certain chemical reactions.
There are few characteristics that nitrous acid have. Although the acid does not fully dissolve in water, when it is in water or nitrate salt, it appears pale blue. In the solution, nitrous acid decomposes steadily into the solution. It is a powerful oxidiser, and if it is in contact with phosphorus trichloride, it explodes.
For further information, links have been placed below. I have few questions regarding nitrous acid due to the limited information. I have been questioning the movement of this compound in relation to individual atoms and the actual size of this molecule.
Further information:
https://www.britannica.com/science/nitrous-acid
http://www.softschools.com/formulas/chemistry/nitrous_acid_uses_properties_structure_formula/232/
http://www.chemguide.co.uk/organicprops/amines/nitrousacid.html
https://pubchem.ncbi.nlm.nih.gov/compound/nitrous_acid
Even though I had previous experiences with computer programming, I have never encountered a complex programming. Therefore, my partner and I had a few difficulties at the beginning of the programming. However, when we started to understand more about the programming, it became easier and interesting. Also, it was quite difficult to calculate the exact sizes and angles of both, bonds and the individual atom.
Fortunately, I was able to gain more knowledge about computer programming and its relation to creation of the molecules and atoms, as I did not realise the relationship between computer and science.
Logo: https://vrmath2.net/sites/default/files/user/u422/logo/Nitrous_Acid_Mole...
- eryu2's blog
- Login or register to post comments
- 4948 reads