Science Assessment Fluorine Blog (By Jasmine Wong)

Introduction
A 3D model of an atom or molecule is to be created using the VRMath-2 Program and a blog is to be published about the chosen atom or molecule for the Brisbane State High School Year 9 Term 3 Aspire Science assessment on reacting matter. The atom which was chosen by my group is Fluorine. In this blog, the following elements will be included: a 3D Atomic Model of Fluorine, a description and explanation of the composition, structure and characteristics of Fluorine and questions my group wish to investigate further. Furthermore, difficulties in mathematics and/or programming and improvements to the atomic model will be included.
Fluorine (F) is the first element in the Halogen group (group 17) in the periodic table (Feldman, R. & Marsden, S., 2015). This non-metallic element was discovered by Georgius Agricola in 1530, which he found in the compound Fluorspar. It was not until 1670 when Schwanhard discovered its usefulness in etching glass. Pure fluorine (from the Latin word 'fleure', which means 'flow') was not isolated until 1886 by Henri Moissan (Royal Society of Chemistry, 2016). Furthermore, Fluorine can either be found in nature or produced in a laboratory. It can be found in various minerals and compounds; with the two main compounds to be Fluorspar (CaF2) and Cryolite (Na3AlF6). To be made in a lab, Potassium Fluoride is to be put through electrolysis with Hydrofluoric acid to create pure Fluorine and other compounds (Feldman, R. & Marsden, S., 2015). Today, Fluorine has many uses and is used in glass etching, uranium purification and rocket fuel (Chem4Kids, 2016). Compounds of Fluorine are also present in fluoridated toothpaste and in many municipal water systems where they help to prevent tooth decay (Feldman, R. & Marsden, S., 2015).
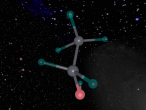
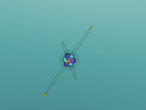
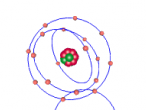
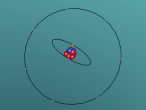
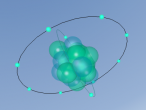
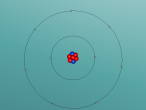
3D Atomic Model
Composition and Structure
Fluorine (F) is the first element in the Halogen group (group 17) in the periodic table (Feldman, R. & Marsden, S., 2015). It has an atomic number of 9 and therefore has 9 protons and 9 electrons. Since it has an atomic mass of 18.998amu, Fluorine has 10 neutrons (Bentor, Y., 2016). There is one more proton than neutrons in the element Fluorine. This is because protons, being charged particles, repel each other. As the element becomes heavier, with each new proton which is added, there is a larger repulsive force. The nuclear force is attractive and stronger than the electrostatic force, but has a finite range. Therefore, it is needed to add extra neutrons, which do not repel each other, to add extra attractive force (Swanson, T., 2016). Fluorine has 2 energy levels; the first energy level has 2 electrons and the second energy level has 7 electrons (Bentor, Y., 2016). Since there are only 7 electrons in the outer shell and the optimal electron configuration of the outer shell contains 8 electrons, Fluorine is an unstable element. Although Fluorine has many isotopes, the only stable isotope found in nature is F-19 (Feldman, R. & Marsden, S., 2015).
Characteristics
Fluorine, the 13th most common element in the Earth's crust, has a density of 1.7g/L. It is in a gaseous state at room temperature and has a melting point of -219.67˚C and a boiling point of -188.11˚C. Fluorine is a very pale yellow-green, dangerously reactive gas. It is the most reactive of all elements and quickly attacks all metals, hence this is why steel wool bursts into flames when exposed to fluorine (Royal Society of Chemistry, 2016). Fluorine is also the most electronegative element, given that it is the top element in the Halogen group and therefore is very reactive. Being the most electronegative element on the periodic table and a Lewis acid in weak acid means that it is a very strong oxidising agent and accepts other elements' electrons when reacting. Fluorine is the most electronegative element because it has 7 electrons in its outer shell. The optimal electron configuration of the outer shell contains 8 electrons, so since Fluorine is so close to the ideal electron configuration, the electrons are held very tightly to the nucleus (Feldman, R. & Marsden, S., 2015). Although this concept is the same for all halogens, Fluorine is still the most electronegative element. This is because as the halogen atoms get bigger (layers of electrons takes up more space), any bonding pair gets further and further away from the halogen nucleus, and therefore is less strongly attracted towards it. In other words, as the atomic number of halogens increase, the elements become less electronegative (Clark, J., 2002).
Questions and Further Information
Although there is a broad range of information about Fluorine which can be found online, I have always wondered what a real atom of Fluorine looked like since it is usually represented in a planetary model with neutrons. I also wonder if a very reactive element, such as Fluorine, can be found in places other than on Earth (such as on other planets, asteroids or in outer space).
For further information, please refer to the following websites:
- http://www.chemicalelements.com/elements/f.html
- http://www.chem4kids.com/files/elements/009_speak.html
- http://www.chemguide.co.uk/inorganic/group7/properties.html
- http://chemwiki.ucdavis.edu/Core/Inorganic_Chemistry/Descriptive_Chemistry/Elements_Organized_by_Block/2_p-Block_Elements/Group_17%3A_The_Halogens/Chemistry_of_Fluorine
- http://www.rsc.org/periodic-table/element/9/fluorine
- http://www.physlink.com/education/askexperts/ae387.cfm
Difficulties and Improvements
When creating a planetary model with neutrons of Fluorine, I had a few difficulties with programming since I have never had experience with it before. On the day when all the Aspire Science classes attended the VRMath-2 3D Modelling Workshop at QUT, I did not understand the codings of the program and therefore received help from a classmate to code the electrons and nucleus. Since her coding was different to mine, as she was doing a different element, I learnt to understand the codings of the VRMath-2 program during the following days. After I had completely understood the coding process, creating a 3D model of Fluorine was reasonably easy.
Although the 3D model of Fluorine was done as accurately possible with the VRMath-2 program, it could have been improved to be more accurate. Instead of programming a planetary model with neutrons of Fluorine, an electron cloud model would have made a more accurate model of Fluorine since electrons do not travel around in a perfect circle. A more accurate model of Fluorine could have also been programmed by calculating the space between the nucleus and each electron shell. Furthermore, since there wasn't much time to learn about the VRMaths-2 program, it was not able to make neutrons and protons vibrate against each other, unlike in a normal Fluorine atom.
Link to 3D Model and Logo Program
The following link will lead to a 3D Model of Fluorine:
The following link will lead to the logo programming of the 3D Model of Fluorine:
Reference List
Bentor, Y. (2016). Periodic Table: Fluorine. Retrieved July 27, 2016 from http://goo.gl/PumlD
Chem4Kids. (2016). Fluor-ine. Retrieved July 25, 2016 from http://goo.gl/xMTKAi
Clark, J. (2002). Atomic and Physical Properties of the Group 7 Elements (The Halogens). Retrieved July 27, 2016 from http://goo.gl/4kcbC
Feldman, R. & Marsden, S. (2015). Chemistry of Fluorine. Retrieved July 25, 2016 from http://goo.gl/qybe0b
Royal Society of Chemistry. (2016). Fluorine. Retrieved July 25, 2016 from http://goo.gl/blH2Ue
Swanson, T. (2016). Question. Retrieved July 27, 2016 from http://goo.gl/oM1QzD
Groups:











Comments
Feedback
Very well structured. Good analysis and description