The Potassium Atom
Potassium is a chemical element classified the atomic symbol 'K' on the Periodic Table. As the seventh most abundant element on Earth (2.6% abundance in Earth's crust), potassium is found in a wide array of industries, despite being commonly known for its integral role as a mineral that allows human cell function and its presence in bananas. Some applications of potassium are (but not limited to) potassium-based fertilisers, pharmaceuticals and manufacturing. Discovered in 1807 by Sir Humphrey Davy, its abundance and simple atomic structure has allowed for exhaustive research and application regarding potassium.
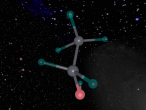
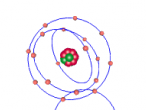
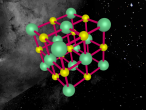
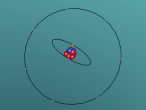
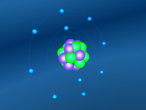
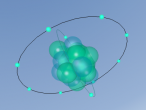
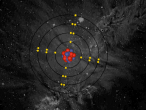
The stable potassium atom is relatively simple. Its composition consists of nineteen protons and twenty neutrons in the nucleus, and nineteen electrons in orbit. The electrons fill the shells in the order of 2, 8, 8 and 1, thus utilising all nineteen electrons in the shell. The above model of the stable potassium atom displays a representation of the atom. However, it should be noted that this model is not to scale. The distance between the electrons and the nucleus is more comparable to the distance between a 10-cent coin and the boundary of a football field. The above model displays electrons in yellow. protons in red and neutrons in blue.
Potassium is often described as a soft, pliable metal. However, potassium can be found in gaseous and liquefied forms as well. The boiling point of potassium is 760 oC and the melting 63.2 oC. The density of the stable potassium atom is .86g/cm3 and the atomic mass is 39.0983 ± .0001u.
Extra information and answers to more complex questions about the fascinating atom of potassium can be found at http://education.jlab.org/itselemental/ele019.html and http://www.rsc.org/periodic-table/element/19/potassium, in addition to slightly less reliable yet detailed sites such as https://en.wikipedia.org/wiki/Potassium.
There were some difficulties during the process of creating the potassium model, albeit it being enjoyable. I was not effectively able to rotate or spin as I wanted it to. I did manage to get some parts to spin, but I had to omit that code because I could not get everything to spin. Additionally, during the process of writing this, attaching a file often caused my work to delete it self. However, this whole experience was overall enjoyable and I gained a deeper understanding into chemistry and programming.