A Neon Atom (Blog)
Chemistry and the idea of matter was first suggested by John Dalton in 1805 in his 'Atomic Theory' and over the century has been expanded on by many other scientists. Chemistry is the scientific category about substances of matter and their properties, compositions and the different reactions that take place when mixed together. Matter has a defined mass, takes up space and is composed of a mixture of elements. An element contains a group of only one type of atom. To identify elements refer to the periodic table which shows all elements with similar atomic structure and there atomic numbers. Atoms are the smallest parts that matter and elements can be divided into and are commonly known as the 'building blocks' of life.
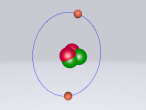
Below is my re-creation of a neon atom.
Structure and Composition of a Neon Atom
Neon also referred to as Ne is a noble gas discovered in 1898 by Sir William Ramsay and Morris Travers at University College London. The pair isolated krypton gas by slowly removing the solid argon, leaving the unknown gas. They named it neon which originates from Greek word 'Neos' meaning new. Neon is a noble gas and is described as odourless and colourless, and unreactive with any other elements. Neon molecules are found with only one single neon atom, meaning the atoms do not group together. Each neon atom contains 10 electrons, 10 neutrons and 10 protons making its atomic number ten. Within the nucleus of a neon atom are the 10 protons and 10neutrons, and together the protons and neutrons give it a relative atomic mass of 20.180. Circling the nucleus are two shells filled with electrons, the first shell containing two electrons, the second with eight. Neon has three isotopes, starting at 20Ne with a relative atomic mass of 19.992, 21Ne with 20.994 and 22Ne with 21.995. Scientists say that neon has no effect on the environment and biological species due to its inert capability to react with other elements and form compounds.
Characteristics of Neon
Neon is the fifth most abundant element in the universe and the second lightest noble gas making it by far the most intriguing element. Neon is however considered a rare gas on earth and found in 1 part to 65,000. Neon is commonly known to be used in 'neon signs' for advertsing, however it can also be used in vacuum cleaners, high-voltage indicators, diving equipment, lasers and switching gears. Pure neon when connected with electricity will glow a reddish orange colour but in most neon signs the substance is mixed with other gases to give it different colour. Neon will boil at a temperature of -246.046 degrees Celsius and melt at a temperature of -248.59 degrees Celsius. It is known to have cubic crystal structure. Neon is known to have no biological role and is considered non-toxic. However if inhaled into the body symptons can be nausea, dizziness and vomitting and air hunger. Oxygen levels can be diminished to 75% of normal levels and can lead to suffocation. However a concentrated amount of neon gas in a contained room is rare and only scientists are exposed to it.
For further, more in depth information and referencing refer to:
- http://www.rsc.org/periodic-table/element/10/neon
- https://www.khanacademy.org/science/chemistry/atomic-structure-and-properties
- https://www2.estrellamountain.edu/faculty/farabee/biobk/BioBookCHEM1.html
- http://www.lenntech.com/periodic/elements/ne.htm
- http://www.chemicalelements.com/elements/ne.html
One small question my research did not take me to is, why in each neon molecule is there only one singular neon atom? Why are there not multiple atoms per molecule? I find this very puzzling as many other element molecules have multiple atoms per molecule. feel free to comment any answers or questions in the comments bar below.
Overall the construction of the neon atom was a fun yet challenging experience on vrmath 2.0 with the help of professional Andy Yeh, QUT science and technology staff and Jade Upton. Learning how to program using logo editor was tricky to get your head around at first but once the basics were taught to us the rest of the construction was fun. The only very frustrating problems I had was following the syntax of programming This programming website is suitable for building simple 3D objects and is recommended for any beginner programmers.
Thankyou for taking the time to read about my experience making a neon atom, hope you enjoyed
Lauren M.
- LAUREN's blog
- Login or register to post comments
- 5164 reads