Methane Molecule Blog
The world is made up of atoms, small tiny building blocks that make up everything that you see. They can be individual or form into compounds and different molecules. My group decided to investigate the molecule methane, by discovering its source and purpose and examining the properties of methane.
Sources of Methane
In nature, methane is produced by anaerobic bacteria which digests organic materials and produce gases such as methane as a waste product. Approximately 80% of methane emissions are sourced from wetlands. The lack of oxygen and abundance of organic matter allow anaerobic bacteria to thrive and produce methane during the process of decomposition of organic material. Furthermore, other natural sources of methane include termites (generated during their digestive process) and oceans.
Methane can also be sourced from the production and combustion of natural gas. It is a primary component of natural gas; which also consists of ethane, propane and other hydrocarbons. Other human-associated activities that contribute to the production of methane include biomass burning, livestock farming and the distillation of coal.
Uses and purposes
The primary use of methane is fuel, in the form of natural gas. Natural gas is distributed through gas pipelines into households for heating and cooking purposes. In addition, it is also used to power gas or steam turbines to generate electricity.
Properties, Composition and Structure
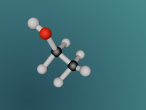
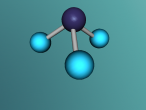
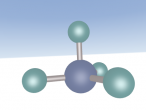
Methane is a colourless and odourless gas with a chemical formula CH4. At room temperature, methane is less dense than air, with a density of 0.717 kg/m3. The melting point is -182.5oC and the boiling point is -162oC. It is also combustible, making methane concentration between 5% and 15% highly flammable and explosive.
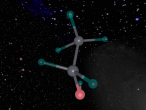
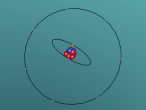
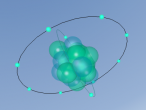
It has a similar shape to a tetrahedron, with the carbon positioned in the center and the other four hydrogen atoms placed in the corners of the tetrahedron. It is comprised of 4 hydrogen atoms and one carbon atom attached through covalent bonds. The hydrogen atoms bond with the carbon atom because the number of outer shell electrons allow them to share electrons. In a carbon atom, there are 4 outer shell electron and in a hydrogen atom, there is one outer shell electron. The four electrons in the outer shell of the carbon is shared with one hydrogen electron. This allows the covalent bond to form a molecule in a stable form.
Questions for further investigation
During my research on the molecule methane, a few questions have come to my attention. Methane has become a major contributing factor in the increase of greenhouse gases present in the Earth’s atmosphere. Consequently, the greenhouse effect absorbs heat energy and traps the heat within the Earth’s atmosphere. If so, is this effect irreversible or will the atmospheric conditions remain permanent? Also, are there any ways to prevent or minimise the impacts of the greenhouse effect?
VRMath Programming
During this enrichment project, I have experienced different learning methods and acquired new skills. The introduction to the VRMath software was stimulating and particularly captivating however, my group experienced a few problems. Using a new technology was particularly challenging as I have never done coding before. The hardest part of this program was positioning the turtle in the correct angle, elevation and side. Inserting codes to move the turtle step by step was difficult to navigate and manipulate. In addition, there were also problems with the order of the code. In various occasions, the order of the coding was confusing and complicated. However, these problems were overcome by the process of trial and error and seeking help from teachers and supporting staff. Overall, it was an interesting workshop and it exposed us to new advanced technologies used to create our own 3D animation.
Here are a few websites that provide further details on methane:
http://itech.dickinson.edu/chemistry/?p=864
http://www.newworldencyclopedia.org/entry/Methane
https://pubchem.ncbi.nlm.nih.gov/compound/methane#section=Top
Reference List:
http://itech.dickinson.edu/chemistry/?p=864
http://www.newworldencyclopedia.org/entry/Methane
https://pubchem.ncbi.nlm.nih.gov/compound/methane#section=Top
http://www.chemguide.co.uk/atoms/bonding/covalent.html
http://www.chm.bris.ac.uk/pt/harvey/gcse/covalent.html
http://chem.libretexts.org/Core/Organic_Chemistry/Fundamentals/Bonding_i...
https://www.britannica.com/science/methane










Comments
Feedback
In-text?
I liked how you went into specifics of covalent bonding