Hydrogen Peroxide

Hydrogen peroxide (H2O2) is a non-flammable colourless liquid with a sharp odour (National Centre for Biotechnology Information, 2016). This substance is manufactured as aqueous solutions of several strengths (Encyclopaedia Britannica, 2016). Identified and isolated in 1818 by Louis Thernard by accident (Hydrogen Peroxide Therapy, n.d.), hydrogen peroxide is common in many aspects of the modern world. It is primarily used in an industrial context as a bleaching agent, a component of rocket fuel, in pollution control treatment and many more ways (Byjus, 2015).
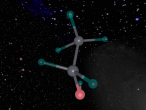
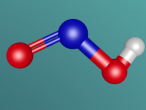
The Hydrogen Peroxide (H2O2) molecule has a non-planar structure (Byjus, 2015) and is comprised of two hydrogen atoms and two oxygen atoms. Hydrogen atoms have 1 valence electron and oxygen atoms have 8 valence electrons. They have covalent bonds which means that they share electrons. Covalent bonds are typically formed between non-metal atoms (BBC, 2014) , to which category both hydrogen and oxygen belong. Because of its covalent bonds, hydrogen peroxide has weak intermolecular forces (BBC, 2014). Hydrogen peroxide is a polar molecule, meaning that the oxygen sections are more negative and the oxygen ends are more positive (W,2011)
The boiling and melting points of hydrogen peroxide are 141C and -11C respectively. It has a highly acidic pH of 1.3. When it combines with other substances, it forms crystalline solids (Encyclopedia Britannica, 2016). Hydrogen peroxide decomposes when exposed to sunlight (Byjus, 2015), as the UV rays catalyse a reaction. This reaction can be expressed by the equation: H₂O₂ →H₂O + O₂.
The following links provide excellent information regarding hydrogen peroxide:
https://www.youtube.com/watch?v=qT3mJrmhJHU
http://puroxi.com/wp-content/uploads/2012/10/Interesting-facts-about-hydrogen-peroxide.pdf
Hydrogen Peroxide by Schumb, Walter C.; Satterfield, Charles N.; Wentworth, Ralph L (http://onlinelibrary.wiley.com/doi/10.1002/jps.3030450224/abstract)
http://rspa.royalsocietypublishing.org/content/royprsa/147/861/332.full.pdf
Overall, I found this programming opportunity to be an excellent one, as I have never had such an opportunity before. Whilst at times the learning got a bit tiring, and once or twice I lost my program due to forgetting to save it, I feel like I have learnt the basics of a skill that may be useful in other fields. Thankfully, I did not choose to program an atom, and therefore did not have to program moving electrons and electron shells.
Links to my project:
Reference List:
National Centre for Biotechnology Information. (2016). Hydrogen Peroxide.
Retrieved 26/07/16 from
https://pubchem.ncbi.nlm.nih.gov/compound/hydrogen_peroxide#section=Top
Encyclopaedia Britannica. (2016). Hydrogen peroxide.
Retrieved 25/07/16 from
https://www.britannica.com/science/hydrogen-peroxide
Byjus. (2015). Hydrogen Peroxide – Structure and Uses.
Retrieved on 24/07/16 from
http://byjus.com/chemistry/hydrogen-peroxide-structure-and-uses/
W,Shane. (2011). H2O2, the wonderful molecule of Hydrogen Peroxide.
Retreived on 27/07/16 from
http://shanew-h202.blogspot.com.au/2011/03/h2o2-wonderful-molecule-of-hydrogen.html
Hydrogen Peroxide Therapy. (n.d.). A Brief History of Hydrogen Peroxide.
Retrieved on 28/07/16 from
https://hydrogenperoxidetherapypages.org/2013/07/22/a-brief-history-of-hydrogen-peroxide/
BBC. (2014). Covalent Bonding.
Retrived on 26/07/16 from











Comments
Feedback
Questions you wish to investigate further?
Good in-text ref and sources used and i liked how you placed a chemical equation in