Carbon Monoxide Blog (By: Devmi Basnayake)
Contents Brisbane State High
1.2.1 Molecular Compounds vs. Molecular Elements
2.0 Display of 3D Molecular Model
3.0 Analysis of Carbon Monoxide
3.2 Structure and Composition of Carbon Monoxide
3.2.1 Atoms within Carbon Monoxide (CO)
3.2.2 Bonds in Carbon Monoxide
3.2.3 Molecular Element or Molecular Compound?
3.3 Characteristics of Carbon Monoxide
3.3.1 Physical Properties of Carbon Monoxide
3.3.2 Physical Properties of Atoms within CO
3.3.2 Where in the Periodic Table of Elements?
4.0 Links for Further Information
5.0 Further Questions to Investigate
6.0 Difficulties with VR Math 2.0 Programming and Mathematics
1.0 Introduction
1.1 Purpose
This blog provides information on the health hazards of carbon monoxide (CO) as well as the characteristics of CO such as its physical properties, structure and composition. Furthermore, this blog analyses the difficulties in programming and mathematics which were identified while constructing the 3D model of carbon monoxide on VR Math 2.0.
1.2 What are molecules?
Molecules are the smallest unit of a compound and consist of the physical and chemical properties/characteristics of a specific compound. (Holistic, 2016) Molecules are formed through the chemical bonding of two or more atoms as a result of electron sharing. (Rouse, 2008) Furthermore, molecules can be broken down into simpler substances. (Holistic, 2016) Additionally, the speed of the molecules within a substance is based on the surrounding temperature. (Rouse, 2008) For instance, if a molecule is placed in a high temperature then the movement of the molecules would be faster as high temperatures increase the kinetic energy within molecules which allows their movement to simultaneously increase. (Rouse, 2008)
1.2.1 Molecular Compounds vs. Molecular Elements
A molecular compound is a molecule consisting of two or more molecules chemically combined and bonded by a covalent bond. (NA,ND) Whereas, a molecular element consists of only one type of element. A few examples of molecular elements are H2 (hydrogen), O2 (oxygen) and nitrogen (N2). (Hill,2011)
Examples of molecular compounds are Water (H2O), Ozone (O3), Calcium Oxide (CaO), Glucose (C6H12O6) and Ammonia (NH3).(Helmenstine,2016)
1.2 What is an Atom?
Atoms can be best described as the basic unit of all matter and it provides elements (consists of ONE TYPE of atom) with their structure.(Sharp,2013) Atoms consist of three subatomic particles known as protons, neutrons and electrons.(Sharp,2013) Both the protons and the neutrons have a similar size, however, the protons have a positive electric charge while the neutrons have a neutral or no electric charge. (Sharp,2013) The electrons are negatively charged and are approximately 1800 times smaller than the protons and neutrons. (ACARA, 2011, p.6&7) Furthermore, both the protons and neutrons are located in the nucleus while the electrons are located in the electron cloud and within the electron shells or energy levels. The neutrons in the nucleus help balance out the electric charge within the nucleus. (ACARA, 2011, p.6&7)
The electrons are attracted to the protons as electrons are negatively charged and protons are positively charged, therefore, the opposite electric charges cause the electrons to be attracted to the protons. This attraction allows in keeping the electrons within the electron cloud and electron shells. (ACARA, 2011, p.6&7)
A few examples of atoms are Boron (B), Argon (Ar), Aluminum (Al) and Carbon (C).
1.1 Carbon Monoxide
Carbon Monoxide is a gas which is extremely difficult to detect as it is colourless, odourless, tasteless and is often mixed with other gases. (U.S. Department of Labor, 2002) Carbon Monoxide or CO is exceptionally dangerous to our health as it is a poisonous gas. (U.S. Department of Labor, 2002)
Carbon Monoxide is found in combustion fumes made by cars, trucks, portable generators, gas ranges and heating systems. (Vermont Department of Health, 2016) CO builds in places with a lack of good, fresh air flow. (Vermont Department of Health, 2016) Moreover, Carbon Monoxide is produced by the process of incomplete burning of a variety of fuels such as natural gas, coal, charcoal, kerosene and wood. (United States CPSC, NA) Additionally, due to the incomplete burning of these fuels CO is existent in exhaust gases of internal combustion engines. Additionally, this molecular compound is also formed in the metabolism of animals, however, in low quantities. (Wikipedia, 2016)
Carbon Monoxide is used to manufacture numerous amounts chemical products which are both inorganic and organic. (Encyclopedia Britannica, 2016) Carbon Monoxide can cause major threats to our health. (Vermont Department of Health, 2016) This is because when CO enters the body (most likely by inhaling) it lowers the body’s capability to deliver oxygen (as it is toxic to haemoglobin) through red blood cells to vital organs; therefore, each organ eventually malfunctions. (Vermont Department of Health, 2016)
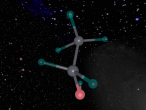
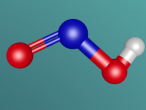
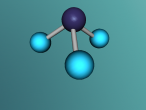
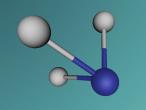
2.0 Display of 3D Molecular Model
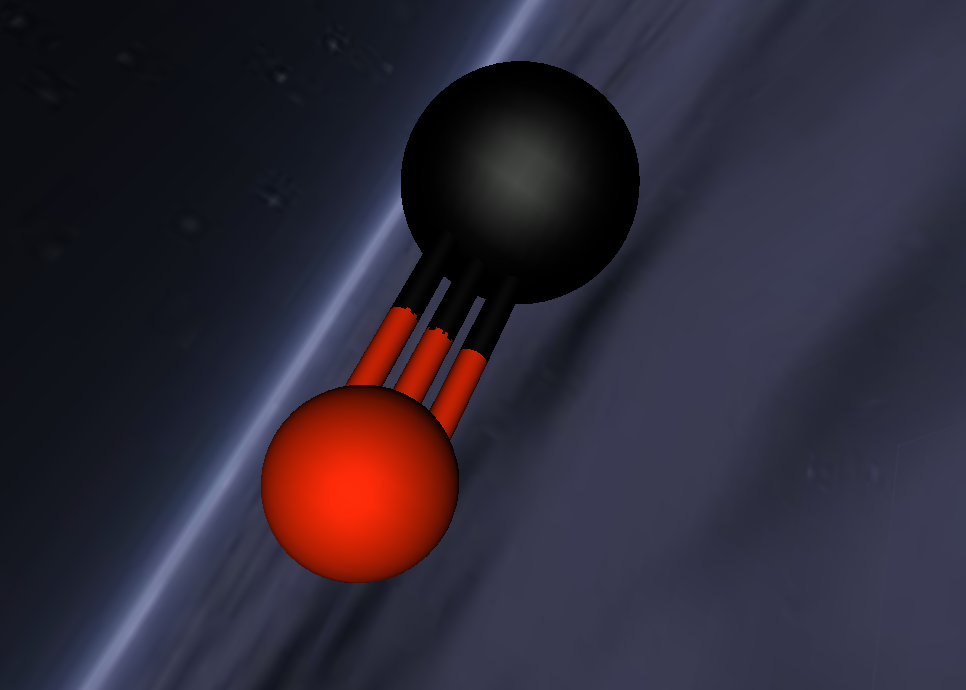
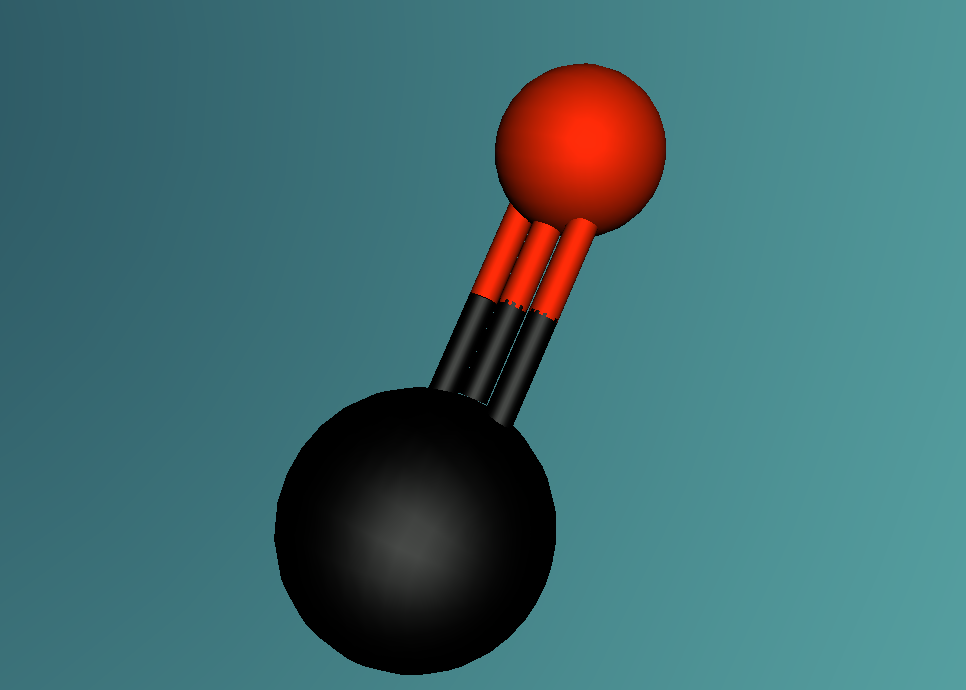
3.0 Analysis of Carbon Monoxide
3.2 Structure and Composition of Carbon Monoxide
3.2.1 Atoms within Carbon Monoxide (CO)
Carbon Monoxide comprises of two atoms, namely, one oxygen atom (O) and one carbon atom (C). (Wikipedia, 2016) Oxygen is a molecular element, this is because oxygen contains two atoms of the same element hence the name O2. (Wikipedia, 2016) Oxygen contains two atoms as one oxygen atom only contains 6 electrons in the outermost shell, therefore, one oxygen atom is unstable. (Bitesize, 2014) Thus, another oxygen atom is needed so both oxygen atoms can share 2 electrons which make 8 electrons on the outermost shell. (Bitesize, 2014)
Carbon is a chemical element which encompasses of 6 neutrons and 6 protons; therefore, there are 6 electrons and 4 electrons in the outermost shell. (Bentor, 2016) Furthermore, there are 5 types of isotopes for carbon, namely, C-11, C-12, C-13, C-14 and C-15. (Bentor, 2016) Carbon is found in food, clothes, cosmetics and gasoline. (EDINFORMATICS, ND)
3.2.2 Bonds in Carbon Monoxide
A covalent bond is formed when two atoms share a pair of electrons.(Clark, 2012) Carbon and oxygen (the two atoms in CO) are held together since carbon is attracted to its electrons while oxygen is attracted to its electrons. (Clark, 2012)
Carbon Monoxide (CO) is triple bonded. It has two covalent bonds, which means there is one electron from carbon and one electron from oxygen pairing up to form a bond. (Wikipedia, 2016) CO also has one dative or dipolar bond. This means that both electrons forming a bond come from one atom. (Wikipedia, 2016) In this case, the dative bond in carbon monoxide contains two electrons from the oxygen atom. (Wikipedia, 2016) This dative or dipolar bond causes a separation of electric charge known as polarisation. (Wikipedia, 2016) This causes a small negative (-) charge on carbon (C) and a small positive (+) on oxygen (O). (Wikipedia, 2016) Due to the opposite charges, oxygen and carbon are attracted to each other. (Wikipedia, 2016) Furthermore, carbon monoxide has 10 electrons in its outermost or valence shell. (Wikipedia, 2016)
3.2.2.1 Why Does CO Have a Triple Bond?
To ensure that both carbon and oxygen atoms have 8 electrons in the outermost shell to make the molecule stable, carbon monoxide consists of a triple bond. The two covalent bonds in the Carbon Monoxide allows carbon and oxygen to have 6 electrons in the valence shell, however, the two elements are still not stable. In order to make them stable 2 more electrons are needed. If there was a third covalent bond then carbon would have 7 electrons and oxygen would have 9 electrons, therefore, a dative bond id needed to allow both elements to have 8 electrons in the outermost shell.
3.2.3 Molecular Element or Molecular Compound?
Carbon Monoxide is a molecular compound. This is because to be categorised as a molecular compound a molecule has to contain two or more different types of elements which are chemically bonded by sharing electrons. (Helmentstine, 2016) Carbon Monoxide has two types of different elements – carbon and oxygen and these two atoms are chemically bonded by two covalent and one dative covalent bond, therefore, carbon monoxide is a molecular compound. (Helmentstine, 2016)
3.2.4 Type of Reaction
Carbon Monoxide is a type of combustion reaction, however, it is an incomplete combustion reaction. (Holt, Rinehart and Winston, NA,p.150) An incomplete combustion is occurred during a combustion reaction, due to a lack of oxygen molecules. (Holt, Rinehart and Winston, NA,p.150) During an incomplete combustion, only a minimum amount of fuel is converted to carbon dioxide (CO2). (Holt, Rinehart and Winston, NA,p.150) The remaining is known as soot or unburned carbon and some carbon monoxide. (Holt, Rinehart and Winston, NA,p.150)
3.3 Characteristics of Carbon Monoxide
3.3.1 Physical Properties of Carbon Monoxide
|
Name |
Carbon Monoxide |
|
Description |
· Tasteless · Odourless · Colourless · Non-corrosive · Highly poisonous · Very flammable |
|
Formula |
CO |
|
Melting Point |
-205.0 degrees Celsius |
|
Boiling Point |
-191.5 degrees Celsius |
|
Density |
1.14kg/cubic metre |
|
Density of Gas |
0.968 |
(COHQ, 2002)
|
Molar Mass |
28.010g/mol |
|
Density |
789 kg/ cubic metre, liquid |
|
Solubility in Water |
27.6 mg/L (25 degrees Celsius) |
|
Types of Liquids Which CO is Soluble In |
· Chloroform · Acetic Acid · Ethyl Acetate · Ethanol · Ammonium Hydroxide · Benzene |
(Wikipedia, 2016)
3.3.2 Physical Properties of Atoms within CO
3.3.2.1 Physical Properties of Carbon
|
Atomic Name |
Carbon |
|
Element Symbol |
C |
|
Group |
14 – Carbon Family |
|
Period |
2 |
|
Block |
P |
|
Melting Point |
3642 degrees Celsius |
|
Boiling Point |
4327 degrees Celsius |
|
Density |
2.26 |
|
Atomic Weight |
12.01 |
|
Electro-negativity |
2.50 |
(Neelands,2010)
3.3.2.1 Physical Properties of Oxygen
|
Atomic Name |
Oxygen |
|
Element Symbol |
O |
|
Group |
16 –Oxygen Family |
|
Period |
2 |
|
Block |
P |
|
Melting Point |
-218 degrees Celsius |
|
Boiling Point |
-183 degrees Celsius |
|
Density |
1.43 |
|
Atomic Weight |
16.00 |
|
Electro-negativity |
3.44 |
(Neelands,2010)
3.3.2 Where in the Periodic Table of Elements?
Carbon Monoxide does not exist in the periodic table as it is not an element but a molecule. However, the elements within this molecule can be identified the periodic table of elements.(Royal Society of Chemistry,2016) Oxygen which is one of the elements which help form CO and is depicted with its element symbol O. (Royal Society of Chemistry,2016) Oxygen (O) is located in Group 16 of the periodic table as it is part of the oxygen family. (Royal Society of Chemistry,2016) Group 16 is also called the chalcogens. Non-metals, metalloids and metals are also present in this group. (Royal Society of Chemistry,2016) On the other hand, carbon is located in Group 14 and is depicted with its element symbol – C. Group 14 is the carbon group. (Royal Society of Chemistry,2016) Similarly to oxygen Group, 14 comprises of non-metals, metalloids and metals. (Royal Society of Chemistry,2016)
4.0 Links for Further Information
For more information on Carbon Monoxide visit these websites below:
|
Content in Website |
URL |
|
CO Properties |
https://pubchem.ncbi.nlm.nih.gov/compound/carbon_monoxide#section=Top |
|
Atoms, Molecules and Covalent Bonding |
|
|
CO and its Health Effects |
http://www.rsc.org/Education/Teachers/Resources/Inspirational/resources/2.7.1.pdf |
|
Carbon Atom |
|
|
Molecules |
|
|
Molecules and Compounds |
http://www.ivyroses.com/Chemistry/GCSE/What-is-a-molecule.php |
|
Atoms |
http://www.qrg.northwestern.edu/projects/vss/docs/propulsion/1-what-is-an-atom.html |
|
CO Production |
5.0 Further Questions to Investigate
· If carbon monoxide is inhaled and causes severe health problems can they be cured? If so how?
· What happens to carbon monoxide when it is placed in an environment with extreme heat?
· What happens to carbon monoxide at -205.0 degrees Celsius? Does it change its form?
· What happens to carbon monoxide at -191.5 degrees Celsius? Does it change CO’s form?
· How can threats from carbon monoxide be minimised?
· How can carbon monoxide be detected?
6.0 Difficulties with VR Math 2.0 Programming and Mathematics
There were numerous difficulties found with both the programming and mathematics in VR Math 2.0, however, these problems were overcome. Another problem encountered while constructing the 3D model of carbon monoxide was adjusting the turtle to the right angle in order to insert cylinders which represent the bonds in CO. However, this problem was soon overcome by using the controls in ‘Quick Command’ panel which helped to position the turtle easily in order to create the bonds. Another issue encountered while designing the CO molecule was the scale.
The mathematics behind the programming was very difficult, however, trial and error were used to get an accurate size. This was especially difficult while setting the scale for the carbon and oxygen atom as the carbon atom needs to be relatively larger than the oxygen atom. Additionally, the mathematics of setting scale was also very difficult during the construction of the cylinders representing bonds as they had to be of an equal size. Furthermore, connecting the two bonds (as one section had to black while the second section had to be red) was also very difficult. This is because when the cylinders representing the bonds were connected they would go into each other; therefore, they had to be adjusted and scaled properly so the cylinders would not intersect.
8.0 Reference List
|
1 |
Australian Curriculum, Assessment and Reporting Authority. (2011). Inside Atoms. Retrieved July 21, 2016 |
|
2 |
Bitesize. (2014). Covalent Bonding. Retrieved July 23, 2016 from http://www.bbc.co.uk/schools/gcsebitesize/science/add_aqa_pre_2011/atomic/covalentrev3.shtml |
|
3 |
Bentor, Y. (2016). Periodic Table: Carbon. Retrieved July 24, 2016 from |
|
4 |
Clark, J. (2012). Co-ordinate (Dative Covalent) Bonding. Retrieved July 24, 2016 from |
|
5 |
COHQ. (2002). Carbon Monoxide – An Introduction. Retrieved July 24, 2016 from |
|
6 |
EDInformatics. (ND). The Carbon Atom. Retrieved July 24, 2016 from |
|
7 |
Encyclopedia Britannica. (2016). Carbon Monoxide. Retrieved 23 July, 2016 from |
|
8 |
Helmenstine, A. (2016). What is a Molecule? – Definition and Examples. Retrieved July 20, 2016 from http://chemistry.about.com/od/moleculescompounds/f/What-Is-A-Molecule.htm |
|
9 |
Helmenstine, A. (2016). What is the Difference Between a Molecule and a Compound? Retrieved July 24, 2016 from |
|
10 |
Hill, N. (2011) What is a Molecular Element? Retrieved July 21, 2016 from |
|
11 |
Holistic, I. (2016). What is a Molecule? Retrieved July 20, 2016 from http://www.ivyroses.com/Chemistry/GCSE/What-is-a-molecule.php |
|
12 |
Holt, Rinehart & Winston. (ND). Reaction Types. Retrieved July 25, 2016 |
|
13 |
NA. (ND). What is the Definition of Molecular Compound? Retrieved July 21, 2016 from https://www.reference.com/science/definition-molecular-compound-ed1405cbe00f77f6 |
|
14 |
Neelands, K. (2010). Periodic Table of Elements. Retrieved July 24, 2016 |
|
15 |
Rouse, M. (2008). Molecule. Retrieved July 20, 2016 from |
|
16 |
Royal Society of Chemistry. (2016). Periodic Table of Elements. Retrieved July 23, 2016 from |
|
17 |
Sharp, T. (2013). What is an Atom? Retrieved July 21, 2016 from |
|
18 |
United States CPSC. (NA). Carbon Monoxide Questions and Answers. Retrieved July 21, 2016 from http://www.cpsc.gov/en/Safety-Education/Safety-Education-Centers/Carbon-... |
|
19 |
U.S. Department of Labor. (2002). OSHA Fact Sheet. Retrieved July 23, 2016 from https://www.osha.gov/OshDoc/data_General_Facts/carbonmonoxide-factsheet.pdf |
|
20 |
Vermont Department of Health. (2016). Carbon Monoxide Poisoning. Retrieved July 23, 2016 from |
|
21 |
Wikipedia. (2016). Carbon Monoxide. Retrieved 24 July, 2016 from |
9.0 Acknowledgements
Queensland University of Technology
VR Math 2.0