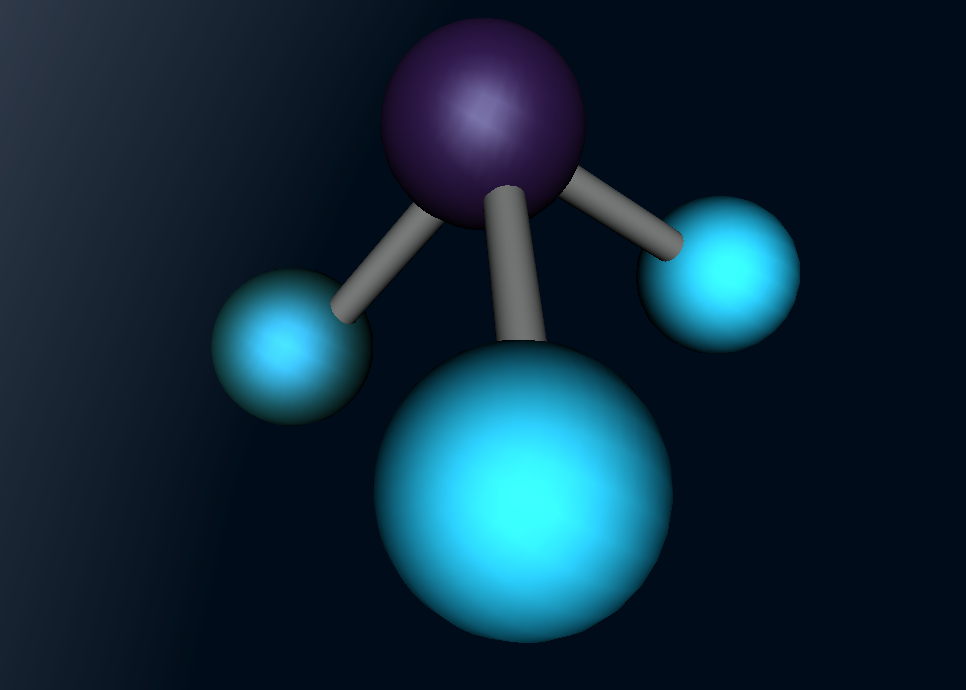
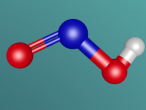
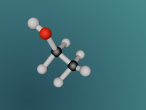
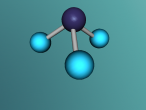
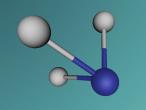
Ammonia (NH3) is a molecule which consists of three hydrogen atoms and a nitrogen atom bonded together. It is a colourless, odorous, alkaline gas which is produced when organic materials decompose. This molecule is essential to many plants and animals, as a source of nitrogen. Additionally, bacteria within the intestines can produce the substance. Ammonia has many uses, though it is mostly used in fertilisers and cleaning products.
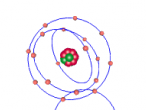
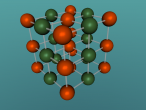
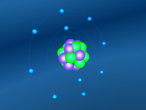
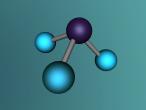
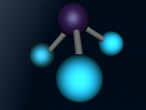
The molecular structure of ammonia consists of three hydrogen atoms forming covalent bonds with one nitrogen atom. Nitrogen is found in group five in the periodic table, whilst hydrogen is in group one. These two elements are in these respective groups because their outer-most rings contain five and one electrons respectively. Owing to the fact that their outer ring has not reached its capacity of electrons, both elements cannot maintain stability on their own. However, by three hydrogen atoms forming covalent bonds with one nitrogen atom, they can form a stable molecule by sharing electrons.
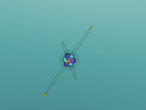
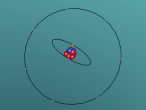
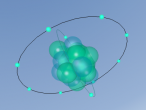
As seen in the diagram above, the outer ring of electrons in the nitrogen atom, symbolised as red circles, is bonding with the three hydrogen atoms. The hydrogen atoms require one more electron each to complete their outer rings and the nitrogen atom requires three more electrons to complete its outer ring of electrons. That is why each of the three hydrogen atoms share their single time electron with the nitrogen atom and the nitrogen atom shares three of its own electrons back to the three hydrogen atoms. This way, the nitrogen atom has the three extra electrons it requires, and the hydrogen atoms have that one extra electron that they each required. The bonds that these atoms make through sharing electrons to form a molecule are known as covalent bonds.
The task of coding that was required to generate the molecular model, as seen below, was rather difficult but also rewarding. It was interesting to discover how typing in various ways commands could make different things happen immediately on the screen. However, the main difficulty that I came across was the fact that if the command was not exactly as it was meant to be, it would not work. For example, I would forget to add a bracket or some minor detail, and such a simple nuance causes the entire model to malfunction. This was quite frustrating, and this project ended up taking up most of the day because of this. Although it was a very tedious process to type the codes perfectly, when it was completed, I was very satisfied with my work as the molecular model because I had successfully accomplished my goal.










Comments
Feedback
I liked how detailed you were with electrons description. However your links are missing and questions for reflection are nowhere to be found.